By Artem Dementyev and Cindy Kao
We visited two lithium-polymer (LiPo) battery manufacturers around Shenzhen: Full River and Shenzhen Sunbang-Power Technology Co., Ltd.
LiPo batteries are commonly used in consumer electronics such as cell phones. One of our projects involve making finger-nail mounted capacitive sensor, NailO (link). The biggest bottleneck to fitting the device into a fingernail was the battery technology. To fit the fingernail form factor we need a very thin (less than 1 mm) and curved battery. Also the battery dimensions need to be smaller than a fingernail: maximum of 15mm x 15mm. We could not find such battery on the market. We were curious why such batteries aren’t common. We started talking to the manufacturers to see if they can make one.
The other option worth mentioning is the futuristic thin-film battery technology. Such batteries are promising since they can be thinner than LiPo (about 0.3 mm).Thin-film batteries have solid electrolyte, unlike the LiPo batteries. This removes the need for a pouch and allows all the layers to be thinly deposited. There are companies such as BlueSpark Technologies and Infinite Power Solutions that make such batteries. Unfortunately, since thin-film technology is immature those batteries are very expensive (about $10 vs. $2 for a LiPo), hard to get, and have a small capacity. It is quite possible that in the near future thin-film batteries will replace LiPo batteries, but for now LiPo is the best option.
We will try to visit a thin-film battery factory soon and write a more informed impression.
The engineers in Full River and Sunbang said that making a thin lithium-polymer battery is challenging for a number of reasons: the assembly would be done by hand, a delicate and time consuming process. The batteries would probably need to be assembled by engineers themselves, instead of the assembly-line workers. Small batteries has stability issues, thus the yield is lower.
Interestingly the battery manufacturing is not as high tech as we thought it would be. The machines are quite simple, and there is a lot of manual work involved.
The manufacturers were very favorable to our request, even though it pushed their manufacturing capabilities.
The battery is composed of five layers: cathode, separator, anode, electrolyte, and the flexible pouch.
LiCoO₂ was the cathode (+) electrode and graphite was the anode (-) electrode. The LiCoO₂ is deposited on a aluminum film and graphite is deposited on copper film. As we understand copper and aluminum are just the conductive structural material on which electrodes are deposited. We are not sure why specifically copper and aluminum were used.
Here are the pictures from the tour with SunBang.
Raw material for the cathode: aluminum foil before it is coated with LiCoO₂.
Graphite (black) is deposited on the copper foil to make the anode.
Raw material: Graphite powder for anode.
The anode and cathode is cut into strips of various widths. The width will determine the size of the dimensions of the battery.
The battery contacts are added in this step.
The long strips of cathode and anode are wrapped together. The white separator is some kind of plastic polymer membrane (imported from Japan, expensive) It is used to separate the anode and cathode, while allowing movement of lithium ions. This process was done by hand or automatically. The automatic is faster but doesn’t support custom sized small batches of batteries. Also it is not possible to use for small batteries.
Wrapping the layers by hand:
Automatic winding with a machine.
The assembly line wrapping the batteries.
Rolled batteries without the pouches.
Batteries are compressed with a press to make them flatter
The batteries are placed inside the pouch with a heat sealer. Pouches are non conductive on outside and conductive inside. The outer layer is nylon, inner layer is a hot melt adhesive membrane, and middle is aluminum. Each battery size requires a custom mold to make the pouch. This step makes it harder for the factory to produce custom sized batteries. The pouches have an extra flap on the side. As we understand this is where the gas produced by the battery goes. The flap is later cut off and the pouch is sealed.
Electrolyte is injected into the pouches manually with a pipette. Electrolyte is composed of lithium based salt LiPF6, dissolved in an organic solvent.
Batteries are charged and tested on large racks. This can take up to 48 hours.
During the testing process, excessive gas is released and goes into the connecting pouch on the left. The left pouch with excess gas is then trimmed off.
Trimming the pouches.
Packaging
Sampled testing: temperature testing. Most batteries are manufactured for -20C to 65C. For extreme climates (e.g., Russia), batteries need to sustain to -40C.
Sampled testing: puncture testing the battery. The machine has a needle that punctures the battery. Company wants to make sure that the battery doesn’t explode or catch on fire when punctured. This test is required for large sized batteries only. Here is a battery after it was punctures.
Finished batteries.
Factory from the outside. The workers live in a dorm on the left, that is right next to the factory.
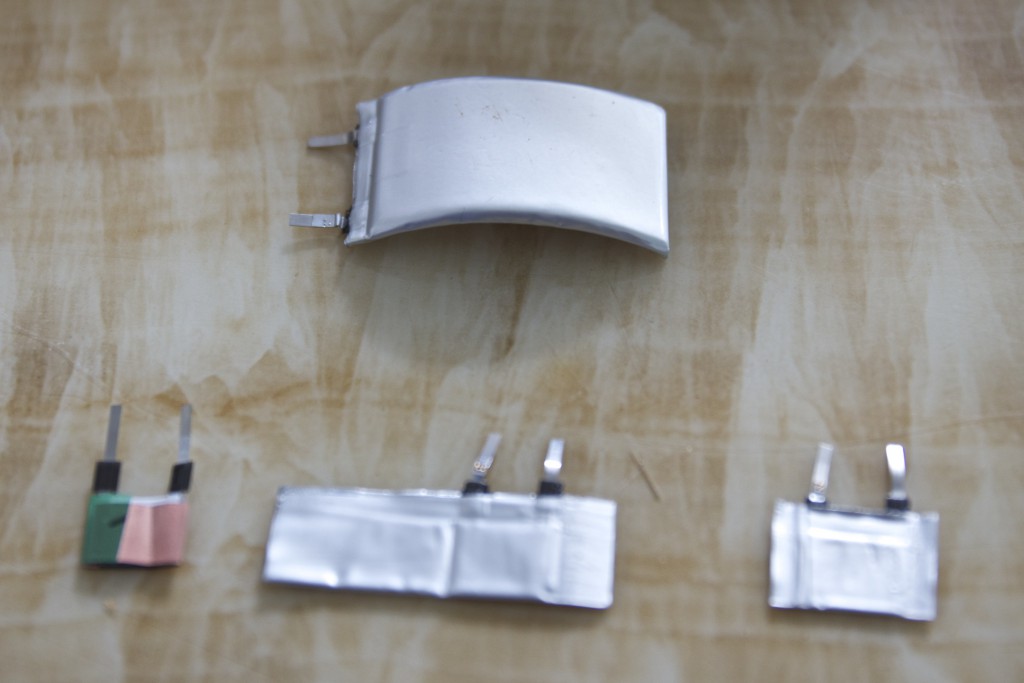
Full River gave us samples of their curved batteries. They also made mock up of the super-thin 0.5mm battery for us.
The thinnest batteries currently in large scale manufacture by Fullriver are ~0.7mm. We are trying to push their limits with 0.5mm.